What did the case report forms say to the research coordinator? You complete me. Valentine’s Day mushiness aside, case report forms (CRFs) are a critical aspect of clinical research. They bring the protocol to life and guide your research staff to properly execute and document your study. A well-written protocol means nothing if you fail to gather the right data to support your hypothesis. In this month’s blog, our Director of Data Management, Robin Solinsky, shares five tips for making CRFs your research team will love.
Study Protocol & CRFs: A Match Made in Heaven
The clinical study protocol governs the first three steps of the scientific method; this is where you identify and scope your research question and develop your hypothesis. The CRFs then govern the last three steps; they provide the mechanism to gather, analyze, and make conclusions about your hypothesis.
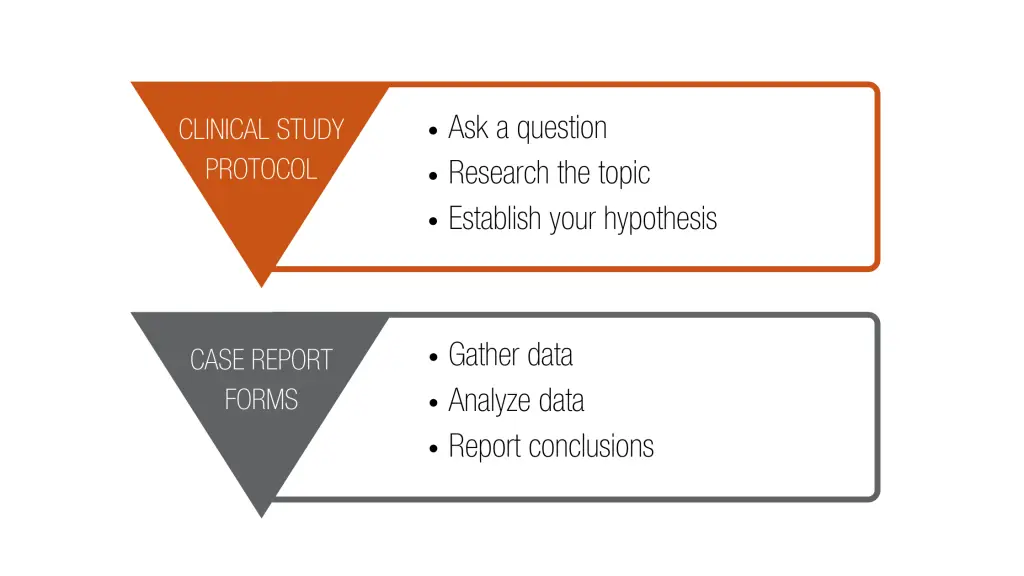
While the protocol is primarily intended to guide the site personnel on study execution, it must also inform regulatory authorities (e.g., FDA), Institutional Review Boards, Ethics Committees, and CMS personnel about your study. To support each of these audiences, the protocol will need to describe the subject population, study methodology, required assessments and their timing, along with important regulatory requirements such as the protection of human subjects.
Whew, that’s a lot of information, and even when the protocol is well-written, it makes for a long, intense document. So, how do you get the data you need in an efficient way? The CRFs are the next step for breathing heart and soul into your study. Let’s dive in and explore how these forms can make your protocol manageable by highlighting critical data in a clear, consistent, and concise format.
1. Complement
The research staff will absolutely read your protocol, front to back. But unless they have a photographic memory, they will not recall every critical detail, and they certainly will not have time to re-read your protocol for every assessment, for every patient, for every timepoint. The CRFs complement the protocol by distilling specific study requirements into smaller logical pieces that facilitate protocol execution and collection of the right data. Using an electronic data capture (EDC) system or other user-friendly database to organize and present your CRFs will help your site with execution, too.
2. Critical
Now, if you recall our blog about the downside of forcing your protocol to collect too much data, the same guidance applies to CRFs. Every data point (i.e., variable) needs to be critical, meaning it falls into one of these two categories:
- Supports an endpoint that informs your hypothesis
- Provides academic data that can be compared to other studies
The adage of “short and sweet” is a good rule of thumb for CRF design and layout. Stay focused on your critical data elements. This allows your research team to get the data you need effectively and efficiently without being bogged down by non-essential assessments. For example, if you only need one qualifying urinalysis within 72 hours of a study procedure, don’t bother asking for all urinalysis results that occur between consent and procedure; collect only the relevant result that supports the eligibility and baseline characteristics. Line items that do not support endpoint or academic needs add noise to the CRFs. For every variable on a CRF, the amount of time spent ensuring its accuracy is ten-fold, which brings us to our next point.
3. Clear
First, here’s a quick review of clinical data flow:
- The research staff captures the data, enters it into a database, and ensures it is accurately transcribed
- Data managers and monitors review the data for completeness, accuracy, and logic (i.e., does this value make sense and does it accurately reflect the medical records?)
- Discrepancies are flagged and the data is clarified or corrected, which means the site edits data and the study team reviews again
- Statisticians evaluate the data and conduct the analysis
Pretty straightforward, but remember, all these steps are done for every critical data point collected for every patient. So, on top of being short and sweet, it is essential that your CRFs are also simple. Make the page easy to read with lots of white space. Identify the critical data to be collected and nothing more. Include the units in which you expect the measures to be collected. When the text fields and labels are clear, you can limit the amount of narrative. In other words, don’t let the site decide or get confused about what kind of data to give you; design the CRFs to call for this information simply, yet specifically.
4. Consistent
Clinical research often requires baseline assessments that are then repeated over time to observe a change. When you design your CRFs in a consistent, predicable manner for each of these repeated assessments, the research staff will know where to put critical data, your monitors will know where to find the data, and your stats team will know how to compare the data.
For assessments that repeat over time, consider making one assessment form that includes the date evaluated rather than calling for the assessment on every follow-up CRF. Also, if a data point does not change with each assessment, there is no value in repeating that field on multiple CRFs. For example, if the randomization arm is recorded on the registration CRF, it does not need to be repeated on procedure or follow-up CRFs. Redundant and illogically or inconsistently organized CRF fields can lead to confusion. What is the real data? Which value is the right one? What will end up in the final report or in manuscripts?
5. Concise
Our final recommendation is to ensure your CRFs are concise. Don’t use them as a dumping ground for checklist items or reminders. Instead, provide the site with separate quick reference guides or program reminder text into the database. For example:
- Do sites need to be reminded to send certain images to the core lab? Instead of asking a question like “Was the imaging sent to the core lab? Yes/No,” program reminder text in the database near the relevant data field. Alternatively, you could collect the date the information was sent to the core lab, but only if the date needs to be tracked and analyzed.
- Is there a lengthy blood sample collection process? Provide the site with a separate “Blood Sampling Instructions” document and let the CRF collect results of the blood sample tests.
A popular saying often attributed to Mark Twain is, “I didn’t have the time to write a short letter, so I wrote a long one instead.” It takes more time up front to design smart and thoughtful CRFs. The investment pays off when your CRFs capture critical data in a format that is easier to complete, monitor, and analyze, thus “completing” your study protocol and saving an exponential amount of time on the back end. Think of your CRFs as your love letter to your research team; they will surely love you in return.