Introduction
Welcome back! As a reminder, part 1 gave us insight in how to define, refine and freeze our clinical study design in ways that align with your overarching product development process. This is essential because your clinical data provides the most influential support for that market authorization, and therefore, your clinical study requires a development process that is carefully planned and robust.
At Bright, we follow a 6-step clinical study development process, so let’s get into the second half of this process. Again, we’ll share several well-honed tips that will increase the likelihood for smooth and successful execution of your clinical study.
The Six Steps of a Clinical Study Development Process
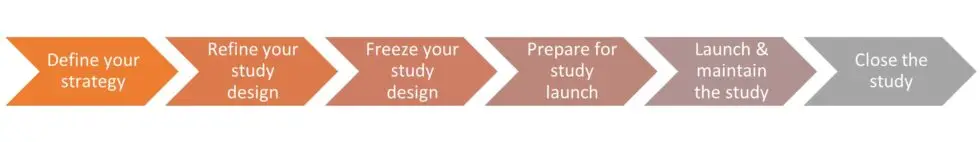
Prepare for Study Launch
IDE approval can occur as quickly as 30 days, especially if you wisely incorporate FDA’s pre-submission feedback. Be sure to keep the investigators and sites apprised of the project status. You’ll want them to know when to expect IDE approval, investigational product availability, and formal site initiation and activation visits.
Remember to communicate study product inventory requirements and replenishment expectations to the manufacturing team. You don’t ever want to allow situations where activated sites with available subjects stall enrollment because the investigational product got held up in your inventory system.
This step is also the time to establish study infrastructure, including the study trial master file and electronic database capture systems, along with associated training.
Launch & Maintain the Study
Great news! With IDE approval, you launched the study at your first site, and they even enrolled the first subject. Better news? You get to do it again… and again, and again. Clinical study execution is a marathon, not a sprint; it takes dedication and stamina to repeatedly launch sites and maintain their engagement. Not all sites start-up at once, and not every site you engage with or even qualify will be activated. Site start-up is a time-consuming and resource intensive effort, so don’t let your foot off the gas. Consider partnering with an experienced team who can focus on making the site start-up experience consistent, efficient, and most importantly, complete.
Just like the product development process, there may be things you learn along the way about the clinical study and its execution, so anticipate changes and be prepared to iterate. In the event of excessive screen failures or slow enrollment, you may need to re-evaluate the eligibility criteria. Perhaps the protocol needs clarification or revision because your monitors found repeated protocol deviations across multiple sites. Whatever the scenario, carefully evaluate the issue and consider the impact of potential changes across the clinical study documentation.
Close the Study
Congratulations, we made it! The sites are monitored and closed, the data is scrubbed clean, and the clinical study report is written. Take your time with the report; this important deliverable is the culmination of everything you’ve planned (and paid) for. Once finalized, you might want to frame the report and hang it on your wall, but you should do something else with it – feed it back into the product development process. Mitigate the risks that only clinical validation could address, update the labeling with the compelling data, and submit your market authorization application – complete with a full, logical, and related data set of bench, biocompatibility, pre-clinical and clinical results. Ah, process… it’s a beautiful thing.
Conclusion
We hope this clinical development process is an intuitive corollary that complements and informs your overall product development process. For more discussion and examples, review our other Bright Insights blog posts on the pre-submission process, smart selection of study endpoints, site selection, and change impact assessments.